

AstraZeneca has admitted for the first time that its Covid vaccine causes a rare side effect.
The pharmaceutical giant admitted that Covishield, which was administered in 150 countries during the pandemic, can cause extremely rare side effects such as blood clots and low platelet count.
The vaccine has been shown to confer substantial protection against Covid-19 in some studies.
However, research has also found an increased risk of a very rare blood clotting condition called TTS, or Thrombosis with Thrombocytopenia Syndrome, following vaccination. This led to restricted use of the AstraZeneca vaccine in a number of countries.
The AstraZeneca vaccine is no longer being offered in the UK, although the Medicines and Healthcare products Regulatory Agency (MHRA) still monitors potential side effects from this vaccine.
It must be stressed that studies overwhelmingly suggest the risk of an adverse event post-vaccination is extremely low.
A 2023 study by the University of Oxford analysed the health records of 29.1 million people in England and estimated that for every 10 million people who are vaccinated with AstraZeneca, there are 66 extra cases of blood clots in the veins and seven extra cases of a rare type of blood clot in the brain.
Infection with Covid-19, in contrast, is estimated to cause 12,614 extra cases of blood clots in the veins and 20 cases of rare blood clots in the brain.
The benefits of getting vaccinated therefore outweigh the risks posed by Covid.
NHS England is urging at-risk groups to get vaccinated against Covid-19 this spring.
The safety of the vaccines has been extensively reviewed in both adults and children by the independent Medicines and Healthcare products Regulatory Agency (MHRA).
AstraZeneca maintains that patient safety remains its top priority and emphasises that regulatory authorities have stringent standards to ensure the safety of vaccines.
AstraZeneca made the seeming U-turn in court documents amid a class action lawsuit filed in the UK over claims that its Covishield vaccine led to deaths and severe injuries. Victims are seeking damages estimated to be worth up to £100million.
One of the complainants alleged that the vaccine caused him a permanent brain injury after he developed a blood clot, preventing him from working.

The pharmaceutical giant is facing a class action lawsuit over claims that its Covishield vaccine led to deaths and severe injuries
GETTY
While AstraZeneca has contested these claims, it admitted for the first time in one of the court documents that the vaccine can “in very rare cases, cause TTS”, or Thrombosis with Thrombocytopenia Syndrome, which is characterised by blood clots and a low blood platelet count in humans.
“It is admitted that the AZ vaccine can, in very rare cases, cause TTS. The causal mechanism is not known,” the company said in the court documents in February, The Telegraph reported.
“Further, TTS can also occur in the absence of the AZ vaccine (or any vaccine). Causation in any individual case will be a matter for expert evidence,” it added.
The admission by AstraZeneca contradicts the company’s previous claim in 2023 that it would “not accept that TTS is caused by the vaccine at a generic level”.
The World Health Organization confirmed that Covishield can have life-threatening side effects.
“A very rare adverse event called Thrombosis with Thrombocytopenia Syndrome, involving unusual and severe blood clotting events associated with low platelet counts, has been reported after vaccination with this vaccine.”
According to the Council for International Organizations of Medical Sciences, “very rare” side effects are those reported in less than one in 10,000 cases.
“In countries with ongoing SARS-CoV-2 transmission, the benefit of vaccination in protecting against COVID-19 far outweighs the risks,” the WHO added.
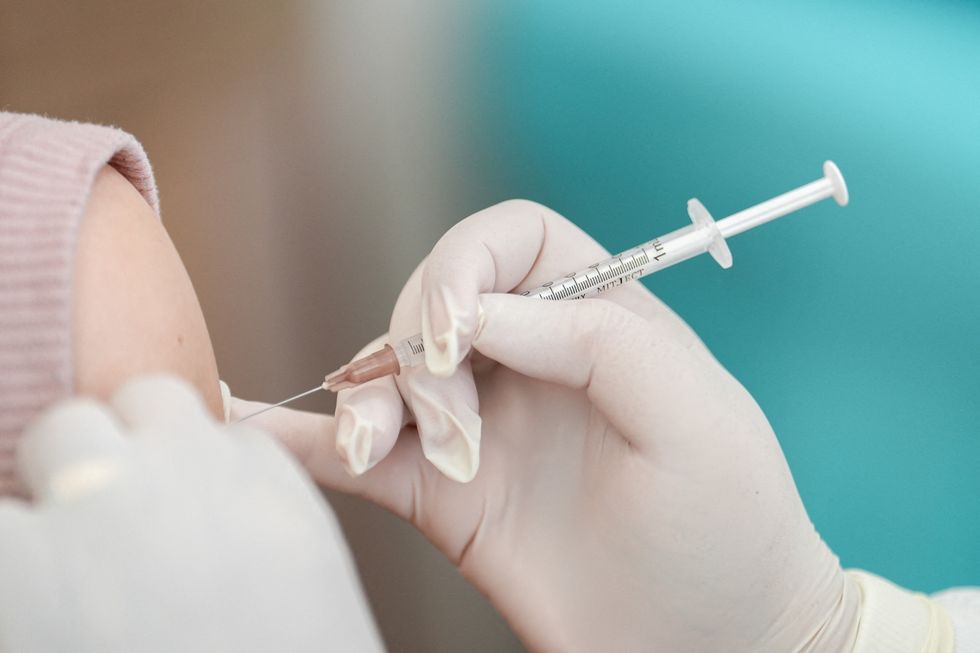
The benefits of getting vaccinated therefore outweigh the risks for the majority of people
Reuters
Editor’s take
The admission is a significant development for victims seeking justice for complications they have developed following Covid vaccination.
However, a sober assessment of the risk to benefit ratio of getting vaccinated must be highlighted here. Context also matters.
The Covid pandemic was a global emergency that required a swift response. Scientists worked with the data they had at the time to constantly learn and adapt to what was a fast moving target.
Independent studies show the AstraZeneca vaccine was incredibly effective in tackling the pandemic, saving more than six million lives globally in the first year of the rollout.
What’s more, authors of a BMJ study noted that the risk of these adverse events is substantially higher and for a longer period of time, following infection from coronavirus than vaccination.
The benefits of getting vaccinated therefore overwhelmingly outweigh the risks for the majority of people.
The NHS national booking system is open for spring Covid-19 vaccination bookings.
People at increased risk from severe illness can get the vaccine, including those aged 75 or over (on 30 June 2024), people with a weakened immune system or who live in an older adult care home.
UKHSA surveillance data on last spring’s programme showed that those who received a vaccine were around 50 percent less likely to be admitted to hospital with Covid-19 from two weeks following vaccination, compared to those who did not receive one.
Spring vaccinations will be available until 30 June 2024.
24World Media does not take any responsibility of the information you see on this page. The content this page contains is from independent third-party content provider. If you have any concerns regarding the content, please free to write us here: contact@24worldmedia.com
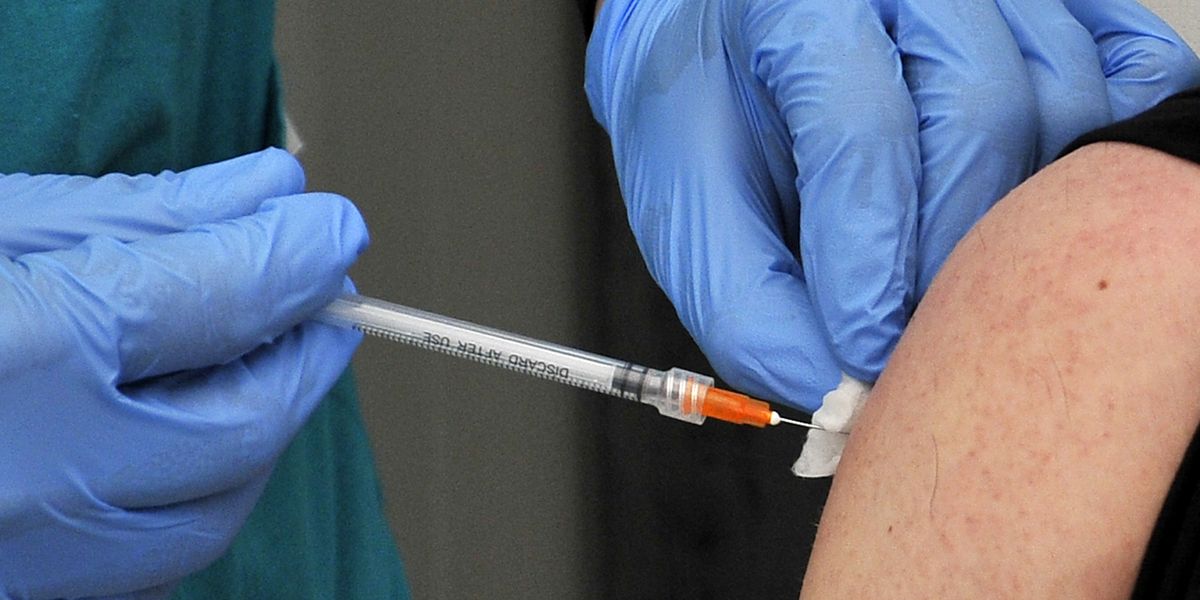
Do you believe the Covid vaccine had negative side effects? VOTE HERE

Latest Google layoffs hit the Flutter and Python groups

‘Women’s rights have been attacked constantly!’

Here’s your chance to own a decommissioned US government supercomputer

AWS S3 storage bucket with unlucky name nearly cost developer $1,300

FTC fines Razer for every cent made selling bogus “N95 grade” RGB masks

Apple confirms bug that is keeping some iPhone alarms from sounding
Roundtables: Inside the Next Era of AI and Hardware

Supplements: Ginkgo biloba boosts memory